Enzyme Mechanisms: Serine Proteases
There are three well known enzymes that go through the serine protease mechanism of action, they are: chymotrypsin, trypsin and elastase. We will look at the enzyme mechanism of chymotrypsin in detail.
A protease is an enzyme that hydrolyzes peptide bonds that link amino acids together in a protein. Proteases are specific for certain amino acids and can hydrolyze those amino acids on the carboxy or amino side of the peptide bond.
There are two general types of proteases:
Endoproteases (Serine Proteases):
Can cleave specific peptide bonds within the protein.
Exoproteases:
Cleave only terminal amino acid residues.
Proenzymes (Zymogens):
An enzyme synthesized initially in an inactive form, but are present and poised to act quickly when needed. Serine Proteases are good examples of proenzymes or zymogens.
Physiological Roles for Proenzymes such as Serine Proteases:
1) Digestion of proteins in the small intestine.
i.e. Trypsinogen (proenzyme) ---------> Trypsin (active form)
2) Blood Coagulation
Chymotrypsin:
>Used as an example of a serine protease because it's structure and mechanism are well understood.
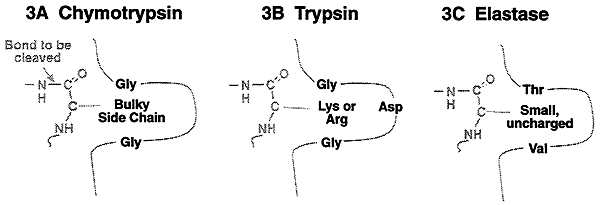
>Catalyzes the hydrolysis of peptide bonds, on the carboxyl side of bulky aromatic side chains (Tyr, Phe, Trp).
Active Site:
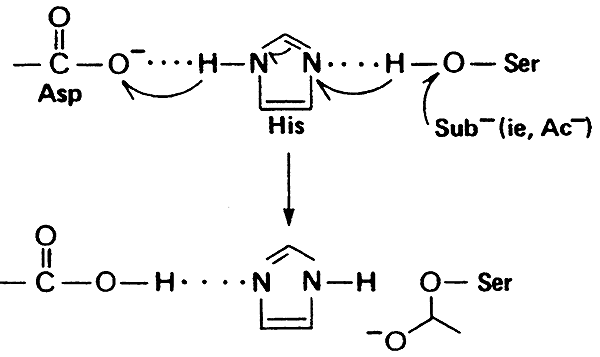
1) Serine, to which the substrate binds, all serine protease active sites contain serine.
2) Histidine, ability to donate and accept protons.
3) Aspartate, ability to accept protons, final link necessary for chymotrypsin action.
Notes:
>These residues are polar (hydrophilic) so would not ordinarily be found on the "interior" of a protein and would normally be deprotonated at physiological pH, except for their critical role in catalysis.
>Though they are in close proximity in the 30 structure, they are not adjacent in the primary sequence (Ser-195, His-57, Asp-102).
Proton Shuttle:
"catalytic triad"

Mechanism of Chymotrypsin Action: "Nucleophilic Acyl Substitution" and "Hydroxylation"
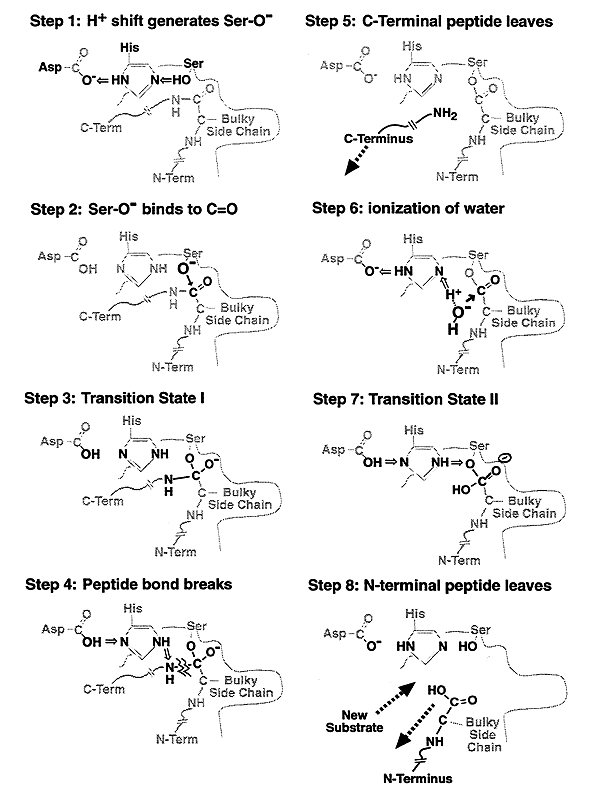
Other Serine Proteases:
>Share the same mechanism of catalysis, but differ in their binding site architecture are trypsin and elastase. Trypsin is specific for the basic amino acids, lysine and arginine. Elastase is specific for the small uncharged amino acids like alanine, valine, threonine etc.
© Dr. Noel Sturm 2014